The Properties of Water POGIL Answer Key unveils the captivating characteristics of H2O, a substance that plays a pivotal role in biological systems and shapes our planet. This comprehensive guide delves into the unique properties of water, exploring its high specific heat capacity, surface tension, and polarity.
Prepare to immerse yourself in a world of scientific discovery as we unravel the secrets of this life-sustaining liquid.
Water’s remarkable properties extend beyond its role in biological systems. Its ability to dissolve a wide range of substances makes it an essential solvent in countless chemical reactions. Understanding the factors that influence solubility is crucial for comprehending the behavior of water in various contexts.
Additionally, water’s acidity and pH play a vital role in chemical reactions and biological processes, shaping the delicate balance of ecosystems.
Properties of Water: Properties Of Water Pogil Answer Key
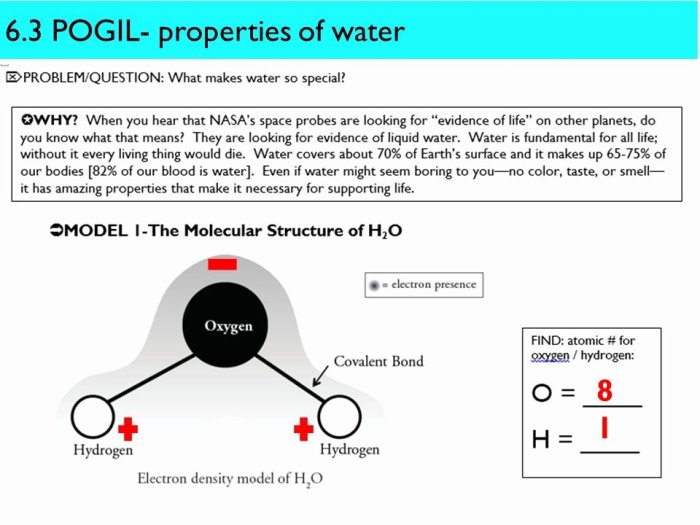
Water, the most abundant substance on Earth, exhibits unique properties that make it essential for life. These properties include its high specific heat capacity, surface tension, and polarity.
Specific heat capacityrefers to the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. Water has a high specific heat capacity, meaning it takes a significant amount of energy to change its temperature.
This property helps regulate the Earth’s temperature, as water absorbs and releases heat slowly, moderating extreme temperature fluctuations.
Surface tensionis the force that causes the surface of a liquid to behave like a stretched elastic membrane. Water has a relatively high surface tension due to the strong cohesive forces between its molecules. This property allows water to form droplets, bead up on surfaces, and support objects that are less dense than it, such as insects walking on water.
Polarityrefers to the separation of electrical charges within a molecule. Water is a polar molecule, meaning it has a slight positive charge at one end and a slight negative charge at the other. This polarity allows water to dissolve a wide range of substances, including ionic compounds and polar molecules.
Cohesion and Adhesion, Properties of water pogil answer key
Cohesionrefers to the attraction between molecules of the same substance, while adhesionrefers to the attraction between molecules of different substances. Water exhibits strong cohesion due to hydrogen bonding between its molecules. This cohesion gives water its surface tension and allows it to form droplets and capillary action.
Water also exhibits adhesion to other substances, such as glass and soil. This property allows water to cling to surfaces and form meniscuses, which are curved surfaces at the interface between water and another substance.
Query Resolution
What is the significance of water’s high specific heat capacity?
Water’s high specific heat capacity allows it to absorb and release large amounts of heat without significant temperature changes, contributing to the stability of biological systems and the regulation of Earth’s climate.
How do cohesion and adhesion affect the behavior of water?
Cohesion, the attraction between water molecules, and adhesion, the attraction between water molecules and other surfaces, influence water’s surface tension and capillary action, shaping its behavior in plants, animals, and various physical systems.
What factors influence the solubility of substances in water?
The solubility of substances in water depends on factors such as temperature, pressure, polarity, and molecular size. Understanding these factors is essential for predicting the behavior of water as a solvent.