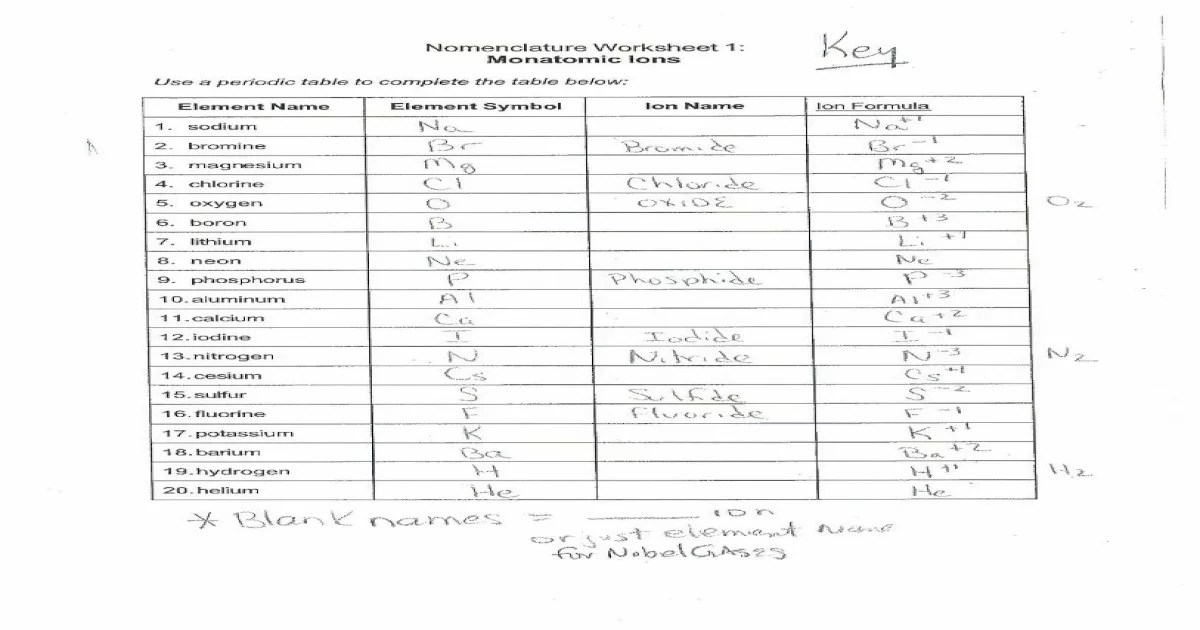
Monatomic ions nomenclature worksheet 1 – Embark on an exploration of monatomic ions nomenclature with Worksheet 1, a comprehensive resource designed to illuminate the fundamentals of naming these essential chemical species. This worksheet provides a structured and engaging approach to understanding the intricacies of monatomic ion nomenclature, empowering you with the knowledge to navigate the world of chemistry with confidence.
Delve into the fascinating realm of monatomic ions, unraveling their unique properties and the significance of their nomenclature in comprehending chemical reactions and processes. This worksheet serves as a valuable tool for students, educators, and practitioners alike, offering a systematic approach to mastering the art of monatomic ion naming.
1. Introduction
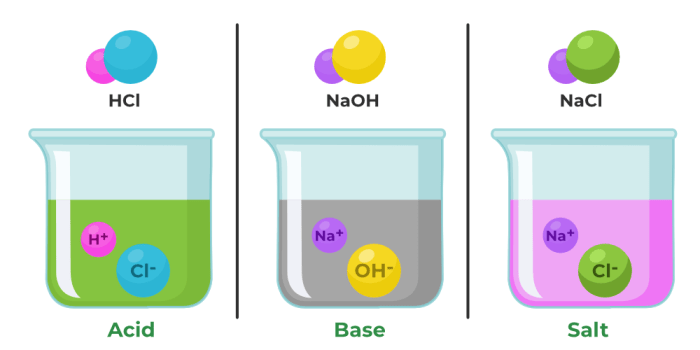
Monatomic ions are charged atoms that have lost or gained electrons. Understanding monatomic ion nomenclature is crucial in chemistry as it allows us to accurately name and identify these ions.
Monatomic ions are classified into cations (positively charged) and anions (negatively charged) based on their charge. The nomenclature of monatomic ions follows specific rules that determine their names and symbols.
2. Types of Monatomic Ions
Cations
- Sodium (Na +)
- Potassium (K +)
- Calcium (Ca 2+)
- Magnesium (Mg 2+)
- Aluminum (Al 3+)
Anions, Monatomic ions nomenclature worksheet 1
- Fluoride (F –)
- Chloride (Cl –)
- Bromide (Br –)
- Iodide (I –)
- Oxide (O 2-)
3. Rules for Naming Monatomic Ions

Monatomic ions are named by combining the root of the element’s name with the suffix “-ide” for anions and the charge of the ion for cations.
For example:
- Sodium (Na) loses one electron to form a cation, Na +, named sodium ion.
- Fluorine (F) gains one electron to form an anion, F –, named fluoride ion.
For ions with multiple charges, Roman numerals are used to indicate the charge. For example, iron can form two cations: Fe 2+(iron(II) ion) and Fe 3+(iron(III) ion).
4. Practice Problems
| Element | Symbol | Charge | Name |
|---|---|---|---|
| Sodium | Na | +1 | |
| Chlorine | Cl | -1 | |
| Calcium | Ca | +2 | |
| Oxygen | O | -2 | |
| Aluminum | Al | +3 |
5. Additional Resources: Monatomic Ions Nomenclature Worksheet 1
- Khan Academy: Naming Ionic Compounds
- ThoughtCo: Naming Monatomic Ions
- YouTube: How to Name Monatomic Ions
Q&A
What is the significance of monatomic ion nomenclature in chemistry?
Monatomic ion nomenclature plays a crucial role in chemistry as it provides a systematic and standardized method for naming and identifying monatomic ions. This nomenclature allows chemists to communicate clearly about these ions, facilitating collaboration and the exchange of scientific information.
How can I use Monatomic Ions Nomenclature Worksheet 1 to improve my understanding of ion naming?
Monatomic Ions Nomenclature Worksheet 1 is designed to provide a structured and interactive learning experience. By working through the practice problems and engaging with the provided resources, you can reinforce your understanding of monatomic ion nomenclature and develop your ability to name ions accurately and efficiently.